Trump has authority to act, and he should.
Send in the Vaccine Troops

The U.S. military can work with the private sector to immunize efficiently.
What happens when one of the best logistics organizations on earth—the United States military—is sidelined in the fight against a pandemic?
On May 15, 2020, President Donald Trump announced the launch of “Operation Warp Speed,” the administration’s public-private partnership to develop, manufacture, and distribute vaccines for Coronavirus Disease 2019 (COVID-19). This disease is a result of infection with the novel coronavirus SARS-CoV-2, which emerged from Wuhan, China in late 2019. On November 9th, just a few months on from the May 15 announcement, pharmaceutical giant Pfizer (and its Germany-based partner BioNTech SE) released the first results of its COVID-19 vaccine candidate. Moderna followed a week later on November 16th.
This astonishingly fast rollout, shortened from the multiple years typical in vaccine development down to just a few months, will be remembered as one of the most remarkable scientific races in modern history. Per the Centers for Disease Control’s vaccine tracker (current as of January 5, 2021), close to 4.84 million people in the United States have received their first dosage of either Pfizer’s or Moderna’s vaccine, with 17 million doses distributed to the states. But this has fallen well short of the ambitious 40 million dose goal initially touted by Operation Warp Speed and the Trump administration as the target for December 31. Though the mainstream media largely continues to spin the delays wholly as an abject failure by the administration, the reality is much more complex than “Orange Man Dumb.”
When the Operation Warp Speed team rolled out their distribution plan on September 16, my immediate commentary focused on two domains: Infrastructure and People. Quite simply, it’s because those two factors are nearly always the single points of failure in any logistical undertaking. Further, risk in supply chains scales exponentially as more complexity is introduced. Availability of raw materials, distance from manufacturing to delivery, human decision making, performance of machines, target markets and demographics, and countless other factors create a fluidity that only the most experienced and creative logistics experts can hope to successfully navigate in real time.
In the specific case of the vaccines produced by Operation Warp Speed, three constraints have emerged as the most troublesome: temperature control, demand planning, and human error. And though the former challenge is predictably manageable when handling any “cold chain” product, it is the latter two issues that can make a logistics planner feel like he’s raising a Mogwai that just won’t stop taking showers and eating after midnight. Unfortunately, the specific framework of OWS’s distribution plan failed to sufficiently account for any of these domains, opening the door for political mischief, narrative war, and an orphan of a plan.
“Not a Day Sooner”
In an open letter released on December 15, 2020, Pfizer CEO Dr. Albert Bourla attempted to assuage concerns about the exceptional requirements imposed on logistics providers and caregivers by his company’s novel vaccine. Instead, he perfectly encapsulated the major chokepoint—a mismatch between need and capacity. His three key points can be paraphrased as follows:
- We revolutionized the way cold chain vaccines are transported and stored by designing improved cold chain packaging,
- We made the distribution process work in a very narrow, time-limited testing phase under controlled conditions, and
- Trust us, we have been doing this a long time.
To the layman, the open letter reads as a reassuring, plausible list of reasons why everything will be just fine. But to a logistics expert—particularly one who has worked in a cold chain logistics operation—it’s a frightening whitewash of a likely failure to take accountability for inevitable issues. This denial of reality is best exemplified by Dr. Bourla’s statement that “our distribution approach involves sending shipments of our vaccine…when they need them, and not a day sooner.”
What Dr. Bourla is saying is that Pfizer planned for a “just in time” supply chain in order to balance manufacturing capacity with the vagaries of transportation capacity, hoping to match production and distribution with the mishmash of next-in-line priorities received from the federal, state, and local governments. Under perfectly optimal conditions, this is a worthy goal. However, even under normal conditions, wastage rates for vaccines can range from 5-10% for vaccines packaged in the 5-10 doses/vial range, as both Pfizer’s and Moderna’s are. It is fair to say that these are less than optimal conditions, logistically speaking. And given the tumult of the post-election interregnum, many of the key stakeholders are wondering if, or how, the problems might ever be fixed.
Ludicrous Speed
When I initially set out to write this piece, I wanted to cover the full domain of issues facing the national vaccine rollout. However, the news space is flooded with these sorts of articles, each saying roughly the same thing: the states are struggling to plan and execute distribution because the federal plan is both too complex and too vague. Another rundown of complaints and data points contributes little and threatens to amplify noise over signal. Rather, I’ll offer what The American Mind is known for: a bold, effective proposal for a major challenge.
Given the confusion and opacity surrounding the Phase 1A allocations of vaccines to each state, and the mixed messages about the role of the military and civilian logistics providers in distributing the Pfizer and Moderna vaccines, it may be best to re-conceive the logistics domain of Operation Warp Speed altogether. The good news here is that this proposal would be workable to implement immediately as a way of getting vaccine distribution back on track.
As of right now, Pfizer and Moderna have both indicated their goal is to produce more than one million doses per day each of their respective vaccines for the United States market through early 2021. This number is likely on the low side, as new contract manufacturers come online and begin churning out vaccines. Nonetheless, it’s a safe production goal to work forward from. Availability of production is the first variable, and is easily determined by run rate at each manufacturing point.
Distribution of need is the second variable, and is a bit hazier. Each state has submitted its own requests to Operation Warp Speed and the manufacturers based on state-level interpretation of CDC’s guidance. Each day, Pfizer and Moderna then have to coordinate shipment of the vaccines to the designated states and distribution hub(s), apportioned by that manufacturing cycle’s availability. As of this moment, manufacturing and distribution are still largely hand-to-mouth, with states requisitioning daily and administering injections as fast as possible.
Analysis of the CDC’s data overlaid with state populations shows that an average of 6.3% of each state’s population has been accounted for, with doses distributed by the manufacturers thus far in Phase 1A and 1B. A range of 5.5% to 7.5% fell within that requisition cycle. Alaska is the outlier at 12.74% of total vaccine requirements already received. Given that distribution of Phase 1A and 1B targets at the state level seems to be roughly in that 5-7% band across the board regardless of how the states are specifically allocating doses, we can begin to form some general macro-level assumptions.
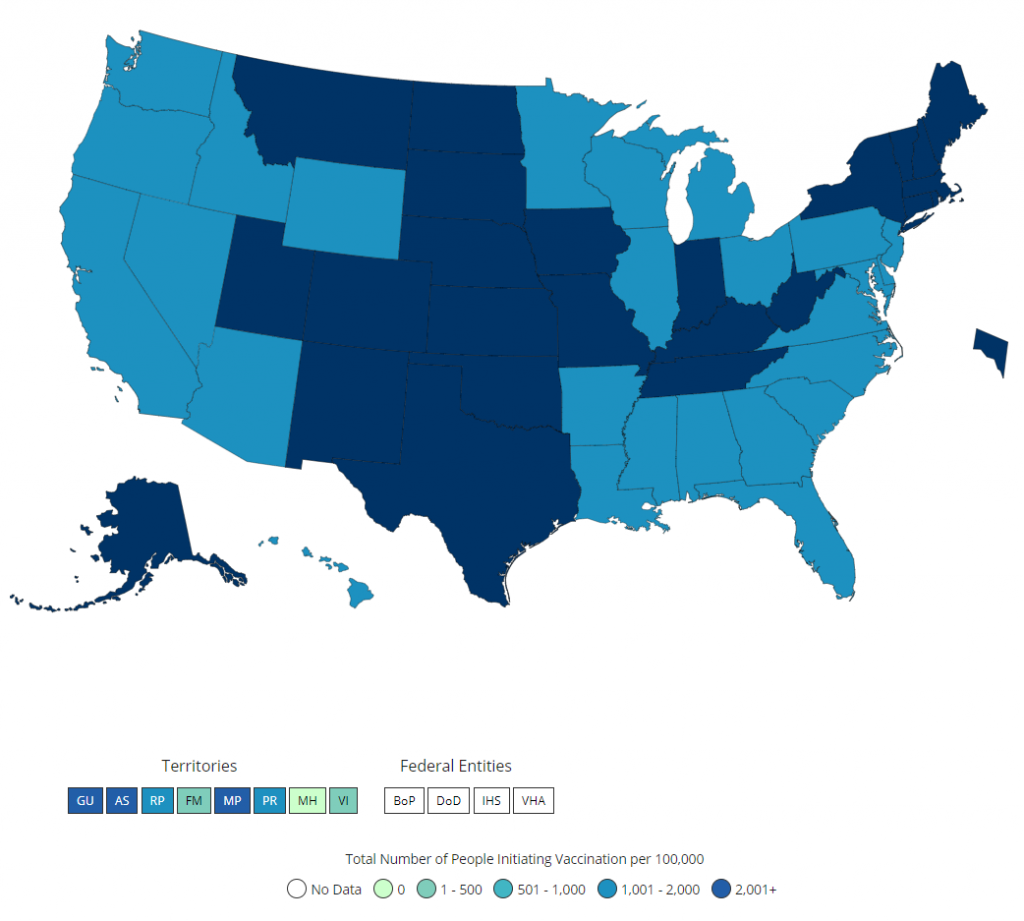
Assumption A is that regardless of the specific demographics of each state in terms of ethnicity, age, income, job, essential or inessential employment, and so on, the states are ultimately receiving about the same percentage of doses relative to their population base. Contra CDC’s extremely complex guidance and models, this heuristic approach has the benefit of maintaining simplicity and avoiding lengthy debates about “who gets what” at a time we should be focusing on speed to market. Assumption B is that we can begin to “chunk” this data down to manageable clusters and build a scalable, high-speed network that focuses on connecting the manufacturers to regional, multi-state hubs, and from there shorten the final mile.
The reason we want to do this is that the current model has too many nodes. Contracting UPS and FedEx as the primary carriers means the vaccines must move through those networks. These are optimized for lowest-cost, highest-speed sorting and distribution of a variety of freight, nearly all of which is non-perishable. And while UPS and FedEx claim to be masters of handling pharmaceutical and time-sensitive cargo, they primarily rely on the performance of the insulated shipping boxes at keeping cargo in good condition while it transits through the regular network.
There is little, if any, direct transportation from vaccine manufacturer to a final distribution hub. More handling inside these logistics networks, then, means more opportunities for loss, breakage, or theft. Further, given the fog of war that naturally occurs during high-volume and high-stress supply chain operations, it is expected that shipments of vaccines are being riskily diverted or re-delivered in real time as a result of demand shift, logistics issues, or outright fraud.
With these assumptions in mind, let’s sketch the distribution requirements. The US is broadly divided into four regions: the South (38% of total population), the West (24%), the Midwest (21%), and the Northeast (17%).

Within these four regions, there’s a decided population skew as well. For example, in the Midwest, Ohio and Michigan alone are 31.7% of the total population for the region. Illinois and Wisconsin make up 27%. In the South, Florida and Georgia comprise 25.6%; Texas holds 23%. In the West, it’s even more stark, with California alone at 50.4% of the entire regional population. And in the Northeast, the three neighboring states of Pennsylvania, New York, and New Jersey are collectively 73.5% of the regional population. As one can see, a vaccine logistics operation could derive precision impact by focusing a majority of its effort on delivering supplies into just these relatively few locales. But how to do that with maximum efficiency and minimum friction?
Fortunately, the United States government already has an inbuilt military and emergency mobilization infrastructure. Recall, the Interstate Highway System was originally built as a dual-use network of road arteries that could allow the military to move supplies and manpower rapidly between population centers in the event of a conflict on U.S. soil. In modern parlance, this capability is known as STRAHNET, or the Strategic Highway Network, which connects certain key military bases to one another via pre-designated routes on the United States’ road network. Conveniently, STRAHNET overlaps neatly with the most efficient means of airlifting and distributing vaccines to a majority of the U.S. population.
With a bit of modification, STRAHNET can overnight become “VAXNET.” In so doing, we greatly mitigate all three constraints: infrastructure, demand planning, and human error. Here’s how we do it.
VAXNET
First, this proposal necessarily makes liberal use of United States military assets and personnel. The United States government has invoked every relevant act or statute—especially the Stafford Act and Defense Production Act—since the beginning of the pandemic to respond to the SARS-CoV-2 outbreak. It beggars belief that we should not treat the vaccine rollout with a similar federalized approach, while still allowing the states and municipalities some flexibility in running their own vaccine campaigns. The key goals of implementing this model are to ease the strain on the domestic logistics network at a time of peak ecommerce logistics demand, streamline the flow of doses from manufacturing sites to the regional hubs, and reduce the number of “just in time” lanes that must be managed over long distances.
Today, the structure of Operation Warp Speed is more akin to drop-shipping to consumers directly from China—it requires high speed, perfect execution, and a lot of luck to operate consistently at scale. Our goal should be to scale the logistics rollout to operate in a similar manner to Amazon’s network.
This operation relies on four lanes of effort working concurrently.
1. Manufacturing and distribution to key military bases by a network of military and commercial aircraft; each base must be equipped with a runway capable of handling medium- and large-body cargo aircraft, sufficient logistics personnel and assets onsite to handle cold chain storage and management of vaccine inventory, and staging of vaccine transfer to commercial carriers for the final mile.
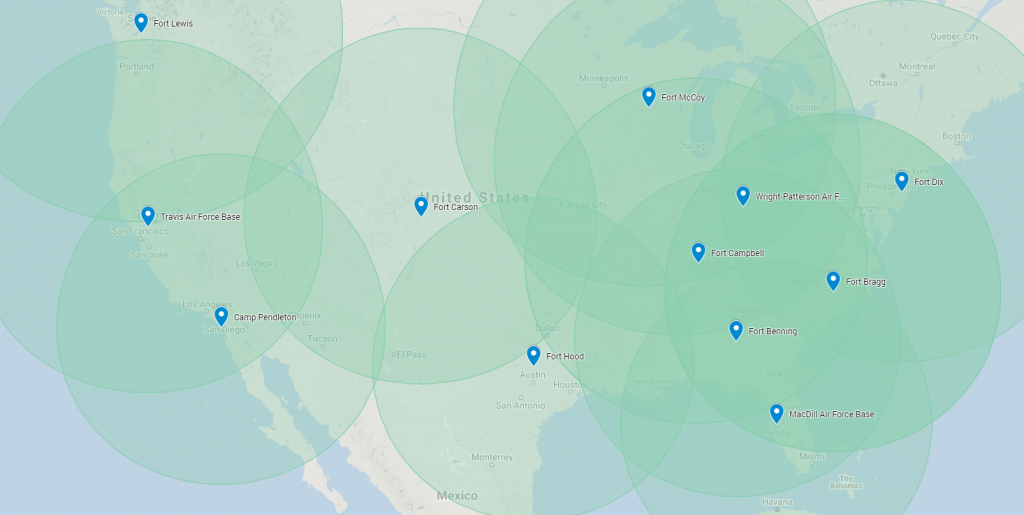
As one can see from these maps, the VAXNET operation endeavors to create overlapping zones of coverage for the most densely-populated areas, based on 600-mile transport zones.
This number is chosen because it is roughly the standard number of miles that can be safely covered by an over-the-road truck driver in given day’s hours of service. Smaller vehicles such as box trucks or sprinter vans can cover even more ground. If team drivers are used, the truck or van need only stop for fuel or food. By airlifting multiple municipalities’ worth of doses from manufacturing to a single hub within one day’s transit to multiple cities or distribution points, the states gain “just in time” levels of flexibility and coordination in allocating doses throughout their areas of responsibility.
Contrast this with the current Operation Warp Speed model of individual cities or states trying to maintain a constant supply chain of doses by relying on UPS or FedEx to deliver in a timely manner, through a complicated feeder network, from much further away.
2. Development of approved lanes from the bases to designated municipal distribution points, with properly-resourced truckload carriers to handle the volume of direct cold chain transport from hub to municipal distribution point.
This is a core competency of many civilian and military logistics professionals, and as such, would not be a difficult task to coordinate immediately. The primary focus must be on clear, frequent communication between the on-base logistics coordinators and the supply chain managers working with each state’s point-of-care service providers in local and rural jurisdictions. Another key focus must be on transparency in demand planning between all stakeholders serviced by each base, and real-time visibility to on-hand and in-transit inventory.
3. “Surge” of training for all caregivers and professionals who may be needed to properly manage the local storage, handling, and injection of the vaccines in their zone.
Logistics can be greatly simplified by understanding a few key heuristics. One I employ frequently is: “Humans are the single point of failure.” While it is true that machines may break down or adverse weather events may occur, it is nearly always human creativity, effort, mistakes, or inattention to detail that determines the success of a supply chain operation.
In the case of vaccine distribution, one emerging bottleneck is an inadequate number of qualified technicians or caregivers to administer the vaccines to recipients. With a reduced burden arising from managing first-mile logistics, state healthcare managers will find additional time and budget to resource the vaccine delivery force. Initiatives could range from up-training paramedics and EMTs, to deploying qualified National Guardsmen, to re-allocating nurses and phlebotomists from COVID-19 testing to vaccine delivery by training secondary staff on the simpler tasks of swabbing and testing patients.
By expanding the pool of qualified and trained vaccine-providing caregivers, states and municipalities can overcome a final-mile bottleneck that can produce huge delays further upstream by delaying timely delivery from hub to arm.
4. Ad-hoc network of vetted and trained pharmaceutical couriers who are capable of transporting small lots of the vaccine (5,000-10,000 doses per trip) from the local storage hubs to point-of-injection sites such as nursing homes, hospitals, schools, or health clinics.
Here is where we draw from the strength of the private sector: individual ambition and technological innovation. The “gig economy,” powered by the rise of Uber, Lyft, eBay, and so many other companies, has created an entire ecosystem of capable, on-demand service providers. These drivers, flippers, handymen, and content creators are hard workers who are used to the shifting demands of a fickle user base. Combined with the existing base of courier companies who specialize in transporting cold chain or “white glove” medical products, the United States can easily scale city- or county-wide efforts to transport vaccines from local hub to injection sites.
Moreover, thanks to the demand-matching capabilities of food delivery and rideshare platforms, the technology exists today to equip a vetted driver with a vaccine delivery unit and allow them to make “milk runs” to multiple facilities from a single hub that scales to the daily demand of each site. By partnering with these courier-enabling services, a national logistics model leveraging centralized hubs can provide high speed, accurate final mile services to any municipality or rural zone.
Note, the goal here is not to perfectly optimize vaccine delivery for every state by diktat from the federal level. Quite simply, a fully nationalized response cannot possibly account for every local variable. If we cast too wide a net, many vulnerable and at-risk citizens will be overlooked. The goal of VAXNET should then be to act as an enabler and force multiplier for the local and state jurisdictions who are closer to the need, and mitigate as many points of friction as possible.
This way, all stakeholders will be free to settle into their niches and begin making decisions based on simple, quantifiable metrics—and not political interest or scarcity. Plainly, federal and state governments always had these resources at their disposal. It was simply a failure of imagination and political will to do it right. Fortunately, it’s not too late.
The American Mind presents a range of perspectives. Views are writers’ own and do not necessarily represent those of The Claremont Institute.
The American Mind is a publication of the Claremont Institute, a non-profit 501(c)(3) organization, dedicated to restoring the principles of the American Founding to their rightful, preeminent authority in our national life. Interested in supporting our work? Gifts to the Claremont Institute are tax-deductible.
Domestic supply chains, not global ones, will save us.
I’m now sure our institutions must be dismantled and rebuilt.
For the first time in centuries, we’re bringing it all back home.




